Do you ever wonder just how many things go to waste in our society of consumption? Are you worried how little care we take of our planet? Are you concerned about global warming issues and the depletion of fossil fuels, set to drive the next generation to a point of no return? Considering that there are so many natural resources around us, we ought to make full use of these by creating green energy for different applications, in a way that cares for our planet as much as possible. First and foremost, the answer to all of these problems lies in electrochemical energy conversion and storage devices.
Electrochemical engineering is a very diverse field in chemical engineering, with applications ranging from fuel cells to batteries and supercapacitors, each covering different categories that can be matched with their corresponding final application. This field is reserved for electricity processes in scaled-up applications, combing the characterization of charge transfer processes at the electrode/electrolyte interphases and materials fabrication and optimization for intded final application. Electrochemistry includes a wide variety of measurements that all correlate, to varying degrees, chemical reactions with how much power and energy can be delivered from such devices . The overarching principle relies on converting the chemical reaction into something useful, for example small-scale electrical energy which can be used for phones and laptops, all the way up to buses and power stations. The concept might sound scary considering our reliance on oil and gas, but hydrogen fuel cell cars and batteries have already been implemented, and they exist across the globe from Japan to the US.
Supercapacitors are one of the forerunners in emerging devices for energy conversion and storage. On the energy-power delivery spectrum, they fall somewhere in between conventional capacitors and fuel cells and batteries. The basic concept of their application is simple: you can create a capacitor by rubbing amber, where electrostatic energy is already stored on the surface. Electrostatic energy is the energy that can be created by separating positive and negative entities, protons and electrons. This separation, created by applying a voltage difference across two separated plates, thereby holding the charges separate from each other, can lead to energy storage. This means that the larger the plates’ surface, the more energy you can store and then supply for your final application.
Now imagine you have a small carbon surface, made up of many different sizes of pits, pores and voids (quite literally millions of them) where the ions can ‘swim’ to in a solution. With this, you would be able to create a massive amount of charge separation by applying just a small voltage difference to power a device application of your choice. Sound a bit confusing? Let’s try out a little thought experiment to understand this: imagine a range of different sizes of voids; big voids can be very easily accessed by ions, at which point these ions can travel from the bigger voids to the smaller ones, which are nestled inside the bigger craters. The smaller cavities can then retain and preserve the ions, which amounts to more charge separation and therefore higher stored energies. An analogy can be drawn between the carbon surface/network and a coral reef arrangement. The bigger craters provide efficient swim-through passages for fish, which in our case can be thought of as the ions, to reach their food which is stored compactly in the smaller holes; a dream-like world of marine fauna brought to life on the microscopic surface of our supercapacitors.
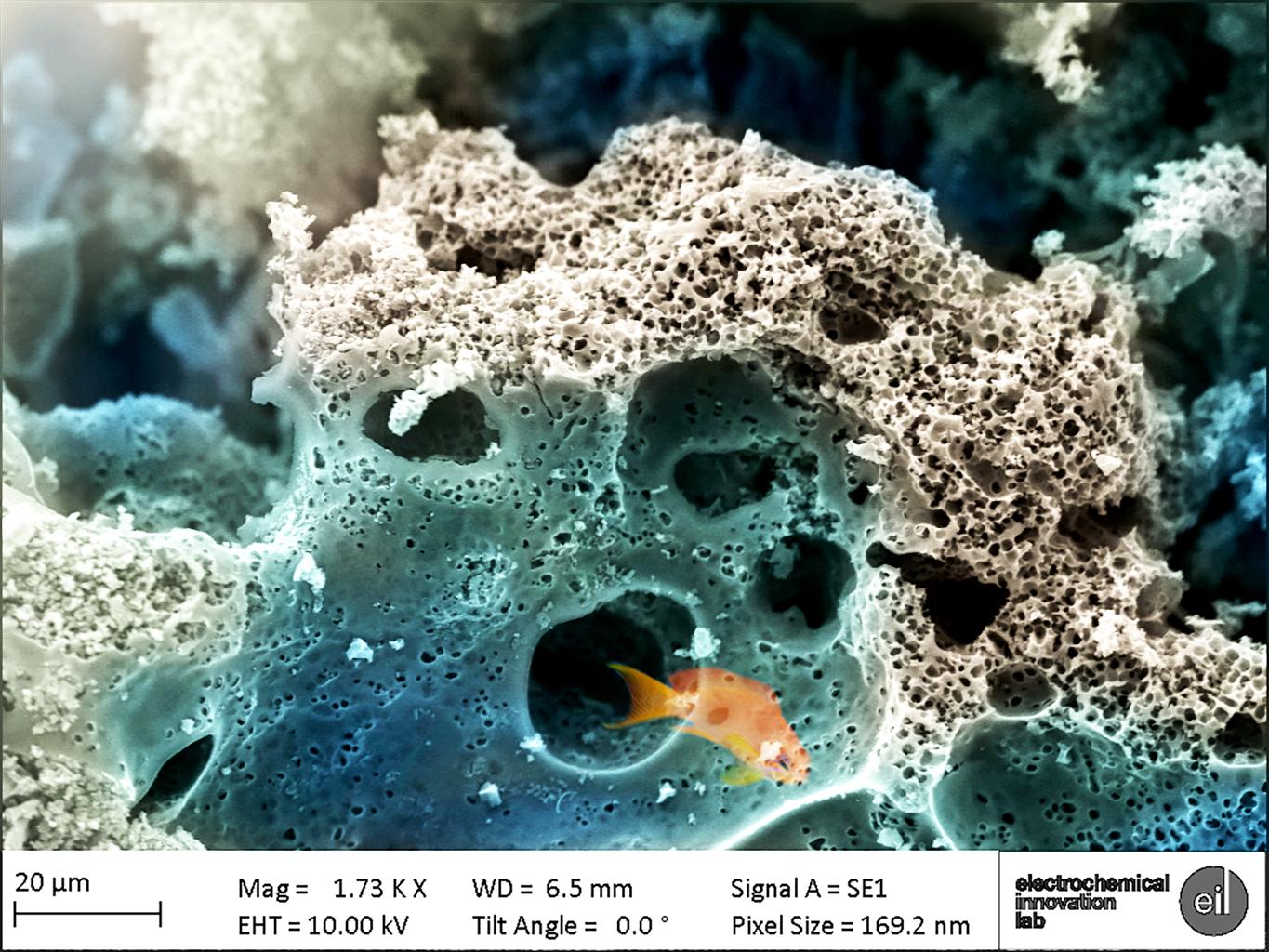 SEM image of porousKOH-activated carbon mimicking aquatic life
SEM image of porousKOH-activated carbon mimicking aquatic life
Now if we go back to a more macroscopic level, we can use these carbon-based devices to hold a vast amount of charge and preserve our environment. Nowadays, researchers are able to use different kinds of raw material, ranging from banana peels, coconut shells and rotten carrots to loofah sponges and tobacco, to fabricate supercapacitor devices. We can utilize numerous waste materials that would otherwise be discarded, by crafting them into an energy device to power our cell phones in just a few milliseconds. Yes, that’s right: the power delivery is so efficient that we can charge our phones incredibly quickly and then return to our game of Candy Crush or figuring out the best way home. High-speed charging will be incredibly useful, considering that everyone is so reliant on their smartphones nowadays.
A new cutting-edge approach now enables us to utilise cellulose in trees, turning it into activated carbon that serves to meet environmental and sustainability requirements. Biocarbon materials, such as cellulose, are predicted to play a leading role in the next generation of materials for many industries due to their abundance, electrical conductivity, low cost and high surface area. Cellulose also has eco-friendly attributes that make it a possible replacement for man-made fibres. The activated carbon is implemented as an electrode (the plate, or carbon surface, of a supercapacitor) for an electrochemical energy conversion and storage cell, which opens the platform for greener electronic and power applications. When mixing the cellulose with different additives, such as potassium hydroxide, we can adjust the void sizes so as to optimize the energy and power delivery; this means that we can store more energy in the phone’s supercapacitor which will be supplied to the phone, thus achieving the highly sought-after rapid charging times.
Combining the cellulose with potassium hydroxide turns it into a very porous, spongy materials when heated, which we need in order to fine-tune all of these voids necessary for charge storage. Balancing the number of voids and their different sizes is key to getting the best performance out of the supercapacitor. The device produced can then achieve safe operations through green chemistry, complementing batteries where high efficiencies, powers and levels of reliability are required.
While each device is distinguished by its own uniquely advantageous characteristics, they all share the motivation of applying greener raw materials and greener application methods to many different final applications. This hybridization of electrochemical cells has been proven to hold great potential for replacing conventional energy sources, powering devices such as phones, wearable flexible electronics and electric vehicles. It is important to note that there is no one device that will achieve all of the advantages we need for a greener environment, or for a faster charging process. Rather, it is the combination of different devices together that will help us achieve the optimal levels of performance. Therefore, it is important to continue to find these innovative solutions, which will lead to increased investment in electrochemical research, paving the way to meet the energy and power demands of the future in a safe, green environment.